Sacrificial Anode Contractors
Cathodic protection works by connecting an external anode to the material to be protected against corrosion and passing an electrical DC current through it. As a result, the entire metal surface becomes cathodic and does not corrode. The external anode can be a galvanic (sacrificial) anode or an impressed current anode, with the current impressed from an external direct current source. Zinc, aluminum, and magnesium are three metals that can be utilized as sacrificial anodes.
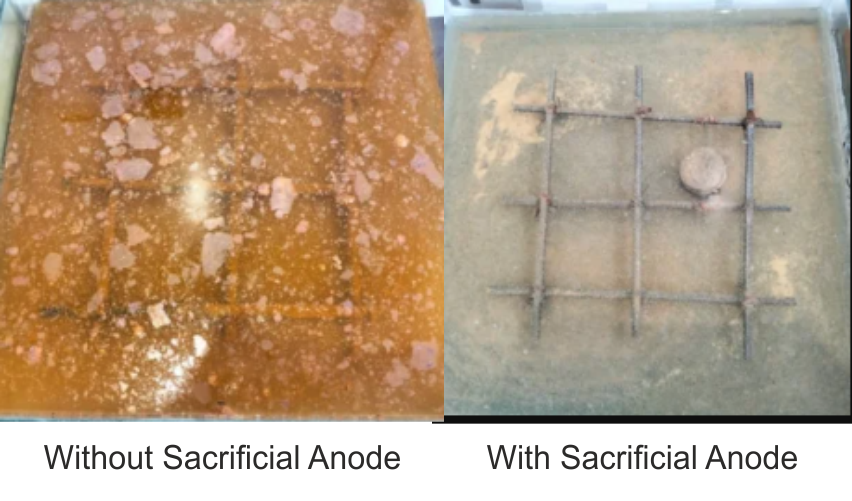
Sacrificial anodes are highly active metals that are employed to prevent the corrosion of a less active material surface. Sacrificial anodes are made of a metal alloy that has a lower electrochemical potential than the metal it is protecting. The sacrificial anode will be consumed instead of the metal it is shielding, hence the name “sacrificial” anode.
Sacrificial anodes are made of either relatively pure active metals, such as zinc or magnesium, or magnesium or aluminum alloys designed expressly for use as sacrificial anodes. A specific backfill material surrounds the anode in applications where the anodes are buried to ensure that the anode produces the desired output.
Recommended Uses:
Sacrificial Anodes are used to protect the hulls of ships, water heaters, pipelines, distribution systems, above-ground tanks, underground tanks, and refineries. Under water / marine rcc structures Concrete floors
